
The U.S. Food and Drug Administration (FDA) doesn’t wait for a single bad shipment to act. If a manufacturer has a history of cutting corners, the agency can shut the door before the next truck even leaves the dock. That’s the power of Import Alerts-a system designed to stop unsafe drugs from ever reaching American pharmacies and patients.
What Exactly Is an Import Alert?
An Import Alert is the FDA’s way of flagging manufacturers, suppliers, or even entire countries as high-risk for drug quality violations. Once a facility lands on an alert list, every future shipment from that source gets automatically detained at U.S. ports without needing to be physically inspected first. This is called Detention Without Physical Examination (DWPE). It’s not a guess. It’s based on patterns: repeated failed inspections, falsified test results, contaminated batches, or missing documentation. The system doesn’t punish one mistake-it targets repeated failure. Since 1995, this tool has evolved into a data-driven engine called PREDICT, which scans over 150 risk factors: inspection history, past refusal rates, product type, and even the importer’s track record. Today, there are 238 active Import Alerts covering everything from antibiotics to cancer drugs. But the most aggressive move came in September 2025, when the FDA launched Import Alert 66-80 targeting GLP-1 receptor agonist APIs-active ingredients used in weight-loss drugs like semaglutide and tirzepatide.The Green List: A New Layer of Control
The September 2025 GLP-1 Import Alert introduced something new: the Green List. This isn’t a blacklist. It’s a whitelist. Manufacturers who prove they meet FDA’s strict quality standards get added to the Green List. Their shipments clear customs within hours. Everyone else? Automatic detention. The distinction is stark. According to CBP data through October 2025, shipments from Green List manufacturers had a 99.2% clearance rate. For everyone else? Just 1.3%. That’s not luck-it’s design. The FDA is forcing manufacturers to prove compliance upfront, not after the fact. And the stakes are high. A single $900,000 shipment from a non-Green List facility can lead to penalties up to $2.7 million if it’s refused, thanks to liquidated damages under 19 CFR § 159.14.Who’s Getting Hit the Hardest?
The GLP-1 crackdown didn’t spread evenly. Of the 89 facilities caught in the alert, 73 (82%) were in India. Nine were in China. Seven were in Europe. The reason? Many Indian API manufacturers had been supplying low-cost bulk ingredients to U.S. compounding pharmacies and online sellers, often skipping proper validation, stability testing, or cleanroom controls. The FDA’s data showed 68.4% of refused GLP-1 shipments contained impurities exceeding international safety limits. But here’s the twist: 22.1% of the refused shipments actually met pharmacopeial standards. They failed because of paperwork-missing Certificates of Analysis, unverified raw material traceability, or auditors who weren’t FDA-recognized. One manufacturer on Reddit lost $1.2 million in 72 hours because their auditor wasn’t on the FDA’s approved list, even though their facility had ISO 9001 certification.
What It Takes to Get Off the List
Getting removed from an Import Alert isn’t a quick fix. The FDA requires four steps: a full facility inspection (minimum five days), a root cause analysis with a detailed corrective plan, three consecutive compliant shipments verified by the FDA, and a signed executive certification. Most companies need more than one try. FDA data shows failed petitions typically require 2.3 additional submissions. Successful applicants don’t just submit documents. They show proof. Video walkthroughs of corrected processes, real-time batch tracking systems, and third-party audits from FDA-recognized bodies are what win approval. Companies using blockchain traceability-like Pfizer with its MediLedger network-achieved 99.8% Green List acceptance. The cost? $200,000 to $500,000 in tech upgrades, plus $45,000-$68,000 for each audit. And the timeline? On average, it takes 11.7 months to get off an alert. That’s longer than Health Canada’s average of 6.3 months.Real-World Consequences
The impact isn’t theoretical. In just six weeks after the September 2025 alert, Novo Nordisk’s manufacturing partners gained 18.7% more market share. Generic drugmaker Viatris reported a $417 million revenue hit in Q3. Pharmacy benefit managers saw compounded GLP-1 prices jump 14.3% in November. The Indian Pharmaceutical Alliance estimates 28,500 jobs are now at risk across 47 facilities. And it’s not just about money. The 90-day destruction window for refused shipments has created dark workarounds. Some companies are paying brokers to falsify export documents just to avoid losing product. The FDA issued Warning Letter 541598 in October 2025 to a Singaporean intermediary caught doing exactly that. Even more concerning: since 2013, the FDA has granted 157 exemptions to enforcement actions. Mylan/Viatris’ endoscopy equipment was exempted 14 times despite ongoing violations. Shilpa Medicare’s diabetes meds were exempted seven times in 2024. These exceptions raise questions about fairness-and whether the system is being used to protect big players while crushing smaller suppliers.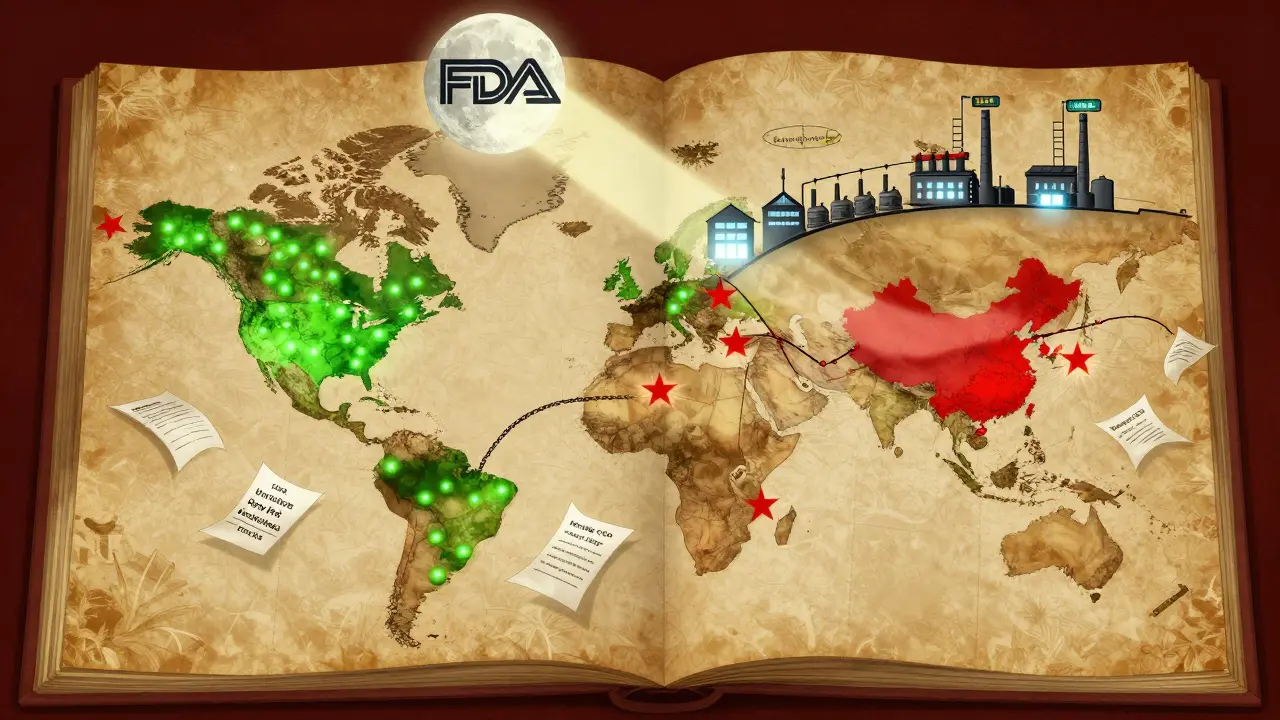
What’s Next?
The FDA isn’t slowing down. In November 2025, they announced expedited Green List processing for facilities using FDA-recognized auditors-cutting approval time from 90 to 45 days. They also launched the API Transparency Portal, giving real-time status updates for 1,842 registered GLP-1 API manufacturers. But bigger changes are coming. FDA Commissioner Dr. Robert Califf confirmed in November 2025 that the GLP-1 alert model will be expanded to all high-risk biologics starting Q1 2026-starting with monoclonal antibodies. That means insulin, cancer drugs, and autoimmune treatments could soon face the same scrutiny. China’s NMPA is already reacting. Starting January 1, 2026, all Chinese API exporters must meet FDA-equivalent facility certifications to sell into the U.S. The European Commission announced similar protocols will be adopted by Q2 2026. The global drug supply chain is being rewired-and only those who invest in transparency, documentation, and traceability will survive.What This Means for You
If you’re a patient: You’re safer. The drugs you get are more likely to be pure, potent, and properly labeled. The days of buying weight-loss injections online with unknown ingredients are ending. If you’re a manufacturer: Compliance isn’t optional anymore. You need to know your suppliers down to Tier 3. You need validated testing, auditable records, and a plan to prove it. The cost of non-compliance isn’t just fines-it’s market exclusion. If you’re a pharmacy or distributor: Verify your API sources. Ask for Green List status. Don’t assume a low price means good value. A $100,000 deal today could cost you $1 million in penalties tomorrow. The FDA’s Import Alert system isn’t perfect. It’s blunt. It’s fast. And it’s working. But it’s also reshaping global pharma. The winners will be those who build quality into their supply chains-not just on paper, but in practice.How does the FDA decide which manufacturers get flagged on an Import Alert?
The FDA uses its PREDICT algorithm, which evaluates over 150 risk factors including past inspection failures, refusal rates, product type, facility history, and importer compliance records. If a manufacturer has multiple violations-like contaminated batches, falsified test results, or missing documentation-the system flags them for automatic detention of future shipments. It’s not based on one incident; it’s about patterns of non-compliance.
What’s the difference between the Green List, Yellow List, and Red List?
The Green List is for manufacturers who’ve proven full compliance and are exempt from automatic detention. Their shipments clear customs quickly. The Yellow List means the FDA has concerns but hasn’t yet issued a full detention order-shipments may still be inspected. The Red List is the most severe: any shipment from these manufacturers is automatically detained without physical inspection. Most GLP-1 API manufacturers are either on Green or Red; Yellow is rare under the new system.
Can a company get removed from an Import Alert?
Yes, but it’s difficult. Companies must complete a full facility inspection, submit a detailed corrective action plan with root cause analysis, prove three consecutive compliant shipments, and get executive certification. Most need multiple attempts. Successful petitions often include video evidence of process changes, not just paperwork. The average time to removal is 11.7 months.
Why are so many Indian manufacturers affected by the GLP-1 Import Alert?
Many Indian API manufacturers supplied low-cost bulk ingredients to U.S. compounding pharmacies and online sellers, often skipping proper validation, cleanroom controls, or full traceability. The FDA found widespread issues with impurities, missing Certificates of Analysis, and unverified raw material sourcing. Of the 89 facilities targeted in the September 2025 alert, 73 (82%) were in India.
What happens to a shipment that gets refused under an Import Alert?
Refused shipments must be exported or destroyed within 90 days under FDA and Customs and Border Protection (CBP) oversight. If the importer doesn’t act, the FDA can authorize destruction at the importer’s expense. Liquidated damages can reach up to three times the commercial value of the goods. For a $900,000 shipment, that means penalties of up to $2.7 million.
Is the Green List system fair to smaller manufacturers?
It’s expensive and complex, which favors large companies. Getting onto the Green List can cost $50,000-$100,000 in audits and testing, plus $200,000-$500,000 in traceability tech. Smaller firms struggle to afford this. Meanwhile, the FDA has granted 157 exemptions since 2013 to large companies like Viatris and Shilpa Medicare, raising concerns about inconsistent enforcement. The system is designed to protect patients-but it’s also reshaping the market in favor of well-funded players.







Cassie Widders
January 11, 2026 AT 21:28So the FDA just locked out a bunch of Indian labs because their paperwork was messy? That’s wild. I get safety matters, but this feels like a bureaucratic hammer.
My cousin works in pharma logistics in Mumbai - she said some shops are now hiring ex-FDA inspectors just to fill out forms right. Like, that’s the new job market.
Konika Choudhury
January 13, 2026 AT 08:47USA thinks it owns the world’s medicine supply now? 73 Indian facilities flagged? Pathetic. You people can’t make your own insulin so you bully others. We make cheap life saving drugs for your country and you call it unsafe? Hypocrites.
Darryl Perry
January 13, 2026 AT 23:37The Green List is a brilliant regulatory innovation. It shifts burden of proof from regulator to manufacturer. This is how modern pharmaceutical oversight should operate. No more reactive enforcement. Proactive compliance. Period.
Windie Wilson
January 15, 2026 AT 09:44So let me get this straight - you’re telling me a $900k shipment got turned away because someone forgot to sign a piece of paper? And now we’re all supposed to clap because the FDA is being ‘tough’?
Meanwhile, my neighbor’s cousin bought semaglutide off Instagram and it worked fine. But hey, let’s burn $2.7 million in penalties because of a missing COA. Truly, the American way.
Amanda Eichstaedt
January 17, 2026 AT 06:36This isn’t just about drugs. It’s about trust. For years, we treated global manufacturing like a discount bin at Walmart - low price, don’t ask questions. Now the bill’s come due.
Patients deserve purity. But manufacturers deserve clarity. The Green List gives both. It’s not perfect, but it’s the first time the system actually rewards good behavior instead of just punishing bad.
I work in supply chain compliance. I’ve seen factories spend years trying to get certified. It’s grueling. But when they finally make it? They become the gold standard. That’s worth it.
Abner San Diego
January 18, 2026 AT 10:46Indian manufacturers are being crushed because they’re cheap. That’s the real story. The FDA doesn’t care about safety - they care about protecting Big Pharma’s profits. Viatris gets 14 exemptions? That’s not oversight. That’s corruption.
Eileen Reilly
January 19, 2026 AT 23:43so like… the FDA is basically saying ‘if you dont have blockchain and a $500k budget you cant make medicine’? lol. also why are we still letting companies like mylan get 14 exemptions? this system is rigged. also i think the green list is just a way to make generic drugs 3x more expensive. who’s gonna pay for that? me. i’m on medicaid.
Monica Puglia
January 21, 2026 AT 18:08Just want to say - this is actually one of the most thoughtful pieces I’ve read on pharma regulation. 💙
It’s easy to get angry at the FDA, but the truth is: people are dying from fake meds. I’ve seen it. A friend’s mom got a batch of insulin that was 12% off potency. She went into DKA. That’s not a paperwork issue - that’s a life issue.
The Green List isn’t perfect, but it’s the best tool we’ve got to stop that from happening again. Small manufacturers? They need help, not hate. Maybe subsidies? Grants? But don’t tear down the system - fix it.
steve ker
January 22, 2026 AT 18:13Let them fail. Global pharma is not a charity. If you can't meet standards, you don't belong in the supply chain. The FDA is not your babysitter. The market will sort it out.
George Bridges
January 23, 2026 AT 06:51Thank you for laying this out so clearly. I’ve been reading about this for weeks and it’s easy to get lost in the numbers. But the human cost - jobs lost, patients waiting, families stressed - that’s what sticks with me.
Maybe the solution isn’t just stricter rules, but more support for smaller labs. Mentorship. Funding. Technical help. Not just punishment.
Rebekah Cobbson
January 23, 2026 AT 07:38For anyone feeling overwhelmed by this - you’re not alone. I used to work for a small API supplier. We got hit by an Import Alert in 2023. Took us 14 months to get off. We had to rebuild our entire QA system from scratch.
But here’s the thing - we came out better. Our error rate dropped 80%. Our clients trust us more. The pain was real, but the growth was real too.
If you’re a small manufacturer reading this: don’t give up. Get help. Find an FDA consultant. Join a trade group. You’re not alone in this.
Audu ikhlas
January 23, 2026 AT 23:03USA thinks it can control the world's medicine because it has the dollar? India made 80% of the world's generic drugs before you started this witch hunt. Now you're destroying livelihoods and calling it 'safety'. You're not protecting patients - you're protecting your own pharma cartel. Pathetic.
Sonal Guha
January 25, 2026 AT 06:0073 Indian facilities. 9 in China. 7 in Europe. And you think this is about safety? Nah. It's about control. The FDA doesn't care about your COA. They care that you're not American. The Green List? It's just a new tariff with a lab coat.